

| https://www2.mst.dk/Udgiv/publications/2018/02/978-87-93614-73-4.pdf |
The Danish Environmental Protection Agency conducted full life-cycle analysis (LCA) of environmental impacts of a range of grocery bag types. LCAs measure the total environmental impacts (such as greenhouse gas emissions) of a product across their full value chain (including inputs needed for their production).
This was quantified for greenhouse gas emissions, as well as a comparison of 'all environmental indicators' which was a combined value for greenhouse gas emissions, ozone depletion, human toxicity (cancer effects), human toxicity (non-cancer effects), photochemical ozone formation, ionizing radiation, particulate matter, terrestrial acidification, terrestrial eutrophication, marine eutrophication, ecosystem toxicity, resource depletion (fossil), resource depletion (abiotic), and water resource depletion.
Values are given relative to a standard LDPE (Low-density polyethylene) single-use plastic bag, with values indicating the number of reuses a given bag would need to result in an equal environment impact. For example, a value of 5 would indicate a bag would have to be reused 5 times in order to have as low an environmental impact as the LDPE bag.
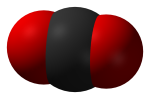
Carbon Dioxide (CO2) is a colorless, odorless gas consisting of molecules made up of two oxygen atoms and one carbon atom. Carbon dioxide is produced when an organic carbon compound (such as wood) or fossilized organic matter, (such as coal, oil, or natural gas) is burned in the presence of oxygen. Carbon dioxide is removed from the atmosphere by carbon dioxide "sinks", such as absorption by seawater and photosynthesis by ocean-dwelling plankton and land plants, including forests and grasslands. However, seawater is also a source, of CO2 to the atmosphere, along with land plants, animals, and soils, when CO2 is released during respiration.

Methane (CH4) is a colorless, odorless non-toxic gas consisting of molecules made up of four hydrogen atoms and one carbon atom. Methane is combustible, and it is the main constituent of natural gas-a fossil fuel. Methane is released when organic matter decomposes in low oxygen environments. Natural sources include wetlands, swamps and marshes, termites, and oceans. Human sources include the mining of fossil fuels and transportation of natural gas, digestive processes in ruminant animals such as cattle, rice paddies and the buried waste in landfills. Most methane is broken down in the atmosphere by reacting with small very reactive molecules called hydroxyl (OH) radicals.
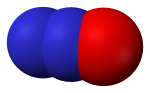
Nitrous oxide (N2O) is a colorless, non-flammable gas with a sweetish odor, commonly known as "laughing gas", and sometimes used as an anesthetic. Nitrous oxide is naturally produced in the oceans and in rainforests. Man-made sources of nitrous oxide include the use of fertilizers in agriculture, nylon and nitric acid production, cars with catalytic converters and the burning of organic matter. Nitrous oxide is broken down in the atmosphere by chemical reactions driven by sunlight.
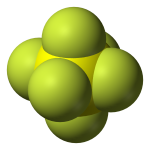
Sulfur hexafluoride (SF6) is an extremely potent greenhouse gas. SF6 is very persistent, with an atmospheric lifetime of more than a thousand years. Thus, a relatively small amount of SF6 can have a significant long-term impact on global climate change. SF6 is human-made, and the primary user of SF6 is the electric power industry. Because of its inertness and dielectric properties, it is the industry's preferred gas for electrical insulation, current interruption, and arc quenching (to prevent fires) in the transmission and distribution of electricity. SF6 is used extensively in high voltage circuit breakers and switchgear, and in the magnesium metal casting industry.
As you might expect from the name, the greenhouse effect works … like a greenhouse! A greenhouse is a building with glass walls and a glass roof. Greenhouses are used to grow plants, such as tomatoes and tropical flowers.
A greenhouse stays warm inside, even during the winter. In the daytime, sunlight shines into the greenhouse and warms the plants and air inside. At nighttime, it's colder outside, but the greenhouse stays pretty warm inside. That's because the glass walls of the greenhouse trap the Sun's heat.

(A greenhouse captures heat from the Sun during the day. Its glass walls trap the Sun's heat, which keeps plants inside the greenhouse warm — even on cold nights. Credit: NASA/JPL-Caltech)
The greenhouse effect works much the same way on Earth. Gases in the atmosphere, such as carbon dioxide, trap heat just like the glass roof of a greenhouse. These heat-trapping gases are called greenhouse gases.
During the day, the Sun shines through the atmosphere. Earth's surface warms up in the sunlight. At night, Earth's surface cools, releasing heat back into the air. But some of the heat is trapped by the greenhouse gases in the atmosphere. That's what keeps our Earth a warm and cozy 58 degrees Fahrenheit (14 degrees Celsius), on average.
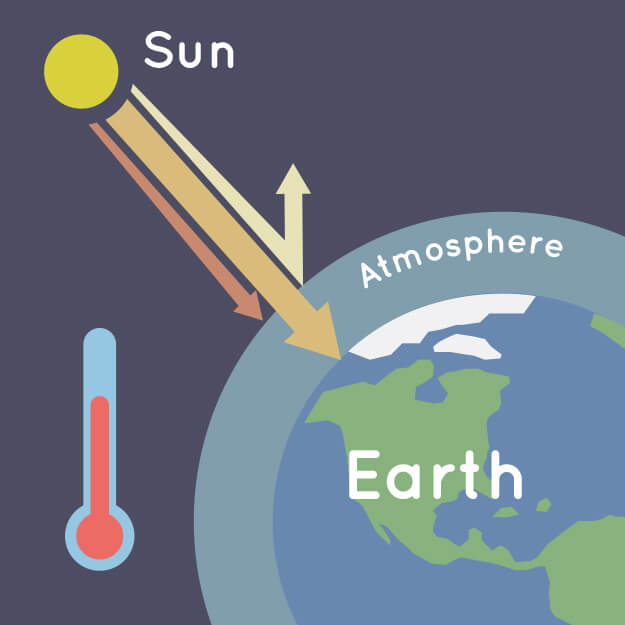
(Earth's atmosphere traps some of the Sun's heat, preventing it from escaping back into space at night. Credit: NASA/JPL-Caltech)